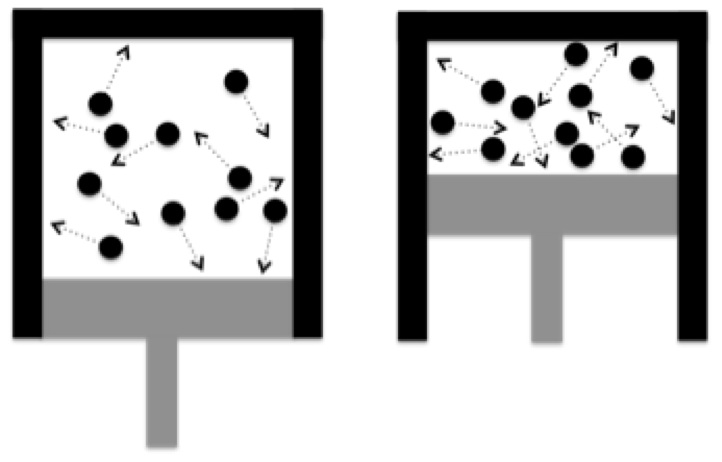
A gas is composed of molecules in constant random motion. Gas will expand to fill and take on the shape of the available space. The molecules are moving at high speed and run into both each other and the walls of any container they are trapped in. The molecules are like BB’s rattling around violently.
The force of the molecules colliding with the surfaces is the pressure inside the container. The more molecules hit the walls, the higher the pressure. So, the more gas you put into the same sized container, the higher the pressure in the container.
The temperature of the gas is a measurement of the speed of the molecules. At absolute zero (-459.67 F) the molecules are not moving at all. The higher the temperature above absolute zero , the faster the molecules are moving when they hit the walls and the higher the pressure in the container.
If you reduce the size of the container, like pushing a piston into a cylinder, then the piston has to run into the molecules to beat them back into a smaller space. This speeds up the molecules causing the temperature to rise. It also increases the density of molecules in the container causing more molecules to run into the walls. The temperature and pressure in the container both increase.
If the size of the container increases, like the gas pushing the piston out of a cylinder, then the molecules lose some of their speed as the piston moves back absorbing some of the molecules energy. This slows the molecules down causing the temperature to fall. The now larger volume in the container reduces the density of the molecules, reducing the number colliding with the walls. The temperature and pressure in the container both decrease.
The Ideal Gas Law expresses the relationship between the pressure, volume, mass, and temperature of gases. The formula is:
PV = mRT
Pressure (P) is the force per unit area the gas molecules put on the walls of the container by colliding with the walls. Volume (V) is how big the container is. The mass (m) defines how many molecules there are. Temperature (T) is how fast the molecules are moving. Since it is based on absolute zero, 459.67 (can round to 460) is added to the Fahrenheit (°F) temperature when used in the formula. The Ideal Gas Constant (R) relates how much thermal energy or work is needed to increase the gas temperature.
The Universal Gas Constant, Ru = 1.986 Btu/lbm°F in thermal terms and Ru = 1,545.35 ft-lb/lbm°F in work terms. The individual gas constant for a particular gas:
RGAS = Ru / MGAS (MGAS is the molecular weight of the gas)
Notice the tight relationship between the thermal characteristics of the gas, like temperature and pressure and the work done by, or on, the gas. If the gas does work on the piston to move the piston, the temperature falls. If the piston does work on the gas to compress the gas then the temperature rises. This relationship is fundamental to thermodynamics. A thermal Btu in work terms is:
1 Btu = 778.2 ft-lb of work.
Since doing 33,000 ft-lbs of work a minute is a horsepower:
Hp = 33,000 ft-lb/min / 778.2 ft-lb/Btu = 42.4 Btu/min or 42.4 * 60 = 2,544 Btu/hr
Converting the heat supplied by the chemical energy stored in fuel into mechanical work is how machines like piston engines and gas turbines “work”.